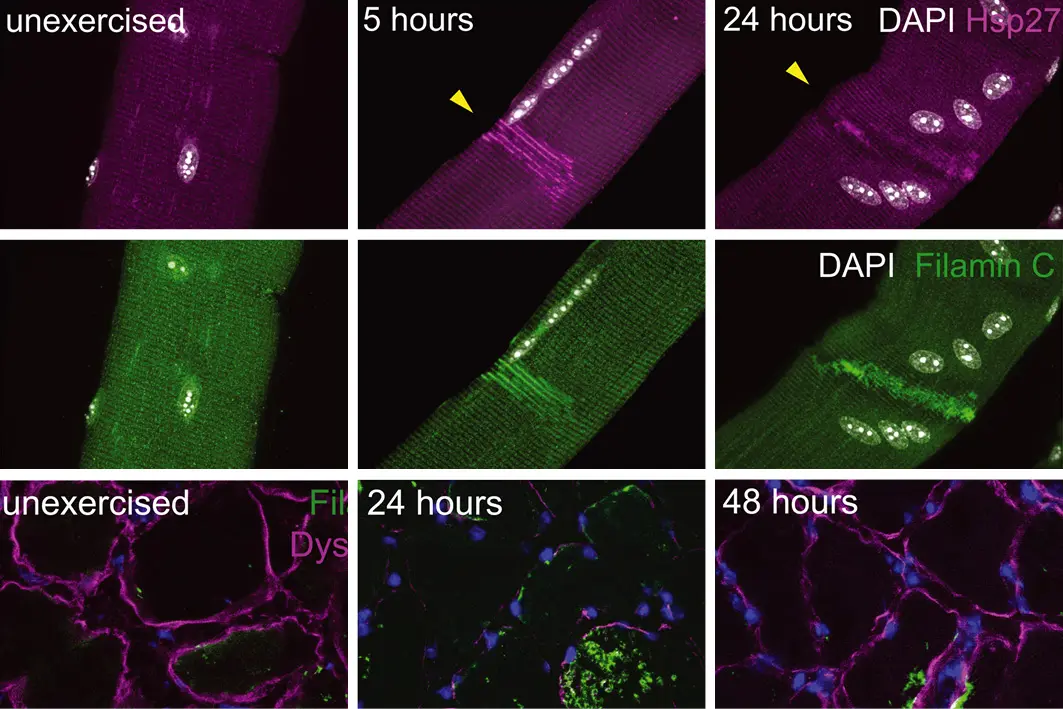
Muscle repair after physiological damage depends on nuclear migration for cell reconstruction
The contractile function of skeletal muscle depends on multinucleated muscle cells (myofibrils), which consist of sarcomeres connected by structural proteins. Despite innate muscle strength and flexibility, mechanical strain and stress can cause muscle damage that is repaired by stem cells (satellite cells). Physical exercise induces mild musculoskeletal injuries that require rapid and efficient repair to maintain muscle homeostasis.
It is generally assumed that homeostasis and regeneration of skeletal muscle depends solely on satellite cells. In contrast to this stem cell-centered view, we have shown that muscle integrity is also maintained through an alternative mechanism of autonomous myofiber repair. Using in vitro, ex vivo, and in vivo models of local muscle damage, we identified nuclear migration as a central mechanism for the rapid local release of mRNA necessary for protein production and repair of damaged sarcomeres.
Skeletal muscle regeneration is a highly synchronized process that requires muscle stem cells (satellite cells). We have found that localized injuries that occur during exercise activate myofiber self-repair, which in mice and humans is independent of satellite cells. Injury to mouse muscle triggers signaling that draws muscle cell nuclei to the injured site via a signaling cascade involving calcium, Cdc42, and phosphokinase C via microtubules and dynein. These nuclear movements accelerate sarcomere repair and locally deliver messenger RNA (mRNA) for cellular reconstruction. Myofiber self-repair is a cell-autonomous protective mechanism and represents an alternative model for understanding the restoration of muscle architecture in health and disease.

Fig. 1 - Myonuclei migrate to local injuries
Roman W, Pinheiro H, Pimentel MR, Segalés J, Oliveira LM, García-Domínguez E, Gómez-Cabrera MC, Serrano AL, Gomes ER, Muñoz-Cánoves P. Muscle repair after physiological damage relies on nuclear migration for cellular reconstruction. Science. 2021 Oct 15;374(6565):355-359. doi: 10.1126/science.abe5620. epub 2021 Oct 14. PMID: 34648328.